- Cisco Community
- Technology and Support
- Security
- Security Knowledge Base
- Cisco ISE Medical NAC Profile Library v2.0
- Subscribe to RSS Feed
- Mark as New
- Mark as Read
- Bookmark
- Subscribe
- Printer Friendly Page
- Report Inappropriate Content
- Subscribe to RSS Feed
- Mark as New
- Mark as Read
- Bookmark
- Subscribe
- Printer Friendly Page
- Report Inappropriate Content
03-14-2016 04:33 PM - edited 08-27-2018 11:12 AM
Caution - Please Read First
This library contains a large number of endpoint profile policies. Before importing to a production ISE deployment, be sure you have read and understand the following conditions and caveats:
- Before importing the profile library to a production ISE deployment, it is highly recommended that you first complete the following tasks:
- Backup the ISE configuration database under Administration > System > Backup& Restore, or via CLI.
- Optionally, export all current ISE profiles under Policy > Profiling > Profiling Policies > Export > Select All.
- Restore current ISE configuration to a lab system and test the import of new profile library. Note resulting profile policy changes to current endpoints which may impact policy assignment in the production deployment.
- The maximum number of profiles that has been QA tested and officially supported for ISE 2.1 and above is 2000. Before importing the new library, check the current number of profiles under Policy > Profiling > Profiling Policies.
This library contains approximately 315 new profiles. Increasing the profiler policy total beyond 2000 profiles (whether via Profiler Feed Service, manual import or custom profile creation) may result in resource capacity issues and service disruption.
- This library is based on Profiler Version 3 compatible updates which ensures that only ISE deployments running ISE 2.1 and above can import the library. This also ensures that each ISE appliance is minimally running 16GB RAM.
- To ensure sufficient memory is allocated to ISE services running on a virtual appliance, verify that your appliance platform is properly detected. There are a couple ways to verify proper the platform detected by ISE.
- From CLI...
ise-node/admin# show tech | begin PlatformProperties
- From Admin UI (ISE 2.2 +)...
Operations > Reports > Diagnostics > ISE Counters > [node] (Under ISE Profile column)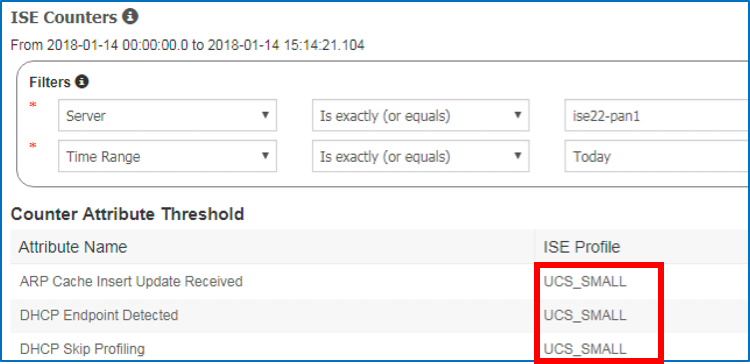
Valid platform sizes include:
- UCS_SMALL
- UCS_LARGE
- SNS_3515
- SNS_3595
- SNS_3595 <super> (ISE 2.4 only)
Any other platform (for example, EVAL, IBM_SMALL_MEDIUM, or IBM_LARGE) will result in insufficient (or less than expected) memory resource allocation for ISE services. Also, make sure that platform detected the platform for which you expect. For example, if deployed 35x5-equivalent VM appliance, make sure it is not displaying as a UCS appliance.
Related defects (Note that many are duplicate or resolved via same patch version):
- ISE VM platform properties defects:
- CSCvd24296 ISE: Revise platform selection rules for ISE installed on VMs
- Fixed In: 2.3P0
- CSCvh71644 VMware OVA templates for SNS-35xx are not detected correctly in platform.properties-active
- Fix: Updated OVAs will be posted to Cisco Software Center for 2.1, 2.2, 2.3, 2.4
- CSCvd24296 ISE: Revise platform selection rules for ISE installed on VMs
- Context Visibility Resource Issues
- CSCvf22318 Exception: All Shards Failed due to "java.lang.OutOfMemoryError"
- Fixed In: 2.1P6, 2.2P4, 2.3P1, 2.4P0
- CSCvf42061 Unable to load Context Visibility all shards failed due to CircuitBreakingException
- Fixed In: 2.1P6, 2.2P4, 2.3P1, 2.4P0
- CSCvh48558 ISE 2.2p5 Unable to load Context Visibility
- Fixed In: 2.2P8, 2.3 P3, 2.4P0
- CSCvg54641 ISE 2.3p1/2.2p4 Unable to load context visibility - java heap size not modified for ibmSmallMedium
- CSCvf22318 Exception: All Shards Failed due to "java.lang.OutOfMemoryError"
- Profiler Feed Service / High Profile Count Issues (Fixed In 2.4 P0)
- CSCvh13873 ISE PSN/PAN App server crashes after profiler feed update
- CSCvh14378 ISE nodes APP Initializing after Feed Service update - out of mem
- CSCvh17860 Profiler Feed Server Proactively taken offline for maintenance
- CSCvh20783 Feed Server undo on PAN does not roll back rules and checks
- Upgrade / Restore issues related to Mem Allocation
- CSCvh57345 Restore of 1.4/2.0/2.0.1 backup fails which taken after Feed update
- Fixed In: 2.2P8, 2.4P0
- CSCvi38845 Upgrade fails after Feed update due to less heapspace
- Requires new Upgrade Bundles to be posted to Cisco Software Center
- CSCvh57345 Restore of 1.4/2.0/2.0.1 backup fails which taken after Feed update
- After installing a large Profile library, be sure to take the following precautions prior to a major ISE version upgrade…
- Backup ISE Configuration database
- Test restore of the ISE configuration to a separate ISE server in a lab environment to verify upgrade process, or else restore to newer version.
- Review the related defects above related to upgrade/restore.
- If you plan to restore existing configuration to a newer version of ISE, be sure you have applied the current patch with the fix for CSCvh57345 on the new ISE PAN node before restore.
- If you plan to use the standard upgrade process, ensure you are using one of the newer upgrade bundles (dated April 2018 or later) which contains the fix for CSCvi38845.
The above steps will ensure that you are not hitting a 2GB Heap memory limitation in upgrade/restore process.
- Logical Profile creation: ISE does not currently support import or API update of logical profiles. Therefore, it is necessary to manually assign the new profiles to a new or existing logical profile. Each of the profiles do have descriptions which can aid in deciding how to logically group the profiles. Each profile can be a member of more than one logical profile. Logical profiles allow groups of devices to be distinguished in Context Visibility and facilitate the creation of policy rules based on logical groupings versus individual profiles.
- When the number of top-level profiles exceeds 500, you will need to switch from Tree-View to List-View to navigate entries beyond the first 500.
Installation
To install, the Medical NAC endpoint profile library:
- Download the Medical NAC library ZIP file
- Unzip the ZIP file on your local computer to get the XML file.
- In ISE, navigate to Work Centers > Profiler > Profiling Policies
- Click Import (
 )
)
- Click Browse...
- Choose the Medical NAC XML file
- Click on Submit.
- Wait 1-2 minutes for the Medical NAC endpoint profiles to be imported!
Once the endpoint profiles are imported, you may view the list of medical devices by choosing Quick Filter and enter "health" under the Description header:

Included Profiles
| 1. | 3M-Device |
| 2. | 3M-Company-Device |
| 3. | 3M-Deutschland-Device |
| 4. | 3M-Germany-Device |
| 5. | Abbott-Device |
| 6. | Abbott-Diagnostics-Device |
| 7. | Abbott-Medical-Optics-Device |
| 8. | Abbott-Point-of-Care-Device |
| 9. | Baxter-International-Device |
| 10. | Gambro-Lundia-Device |
| 11. | Baxter-Healthcare-Device |
| 12. | Beckman-Coulter-Device |
| 13. | Beckman-Lab-Automation-Device |
| 14. | Bosch-Device |
| 15. | Robert-Bosch-Healthcare-Device |
| 16. | Robert-Bosch-Healthcare-Germany-Device |
| 17. | Robert-Bosch-Healthcare-Systems-Device |
| 18. | Danaher-Device |
| 19. | Danaher-Motion-Kollmorgen-Device |
| 20. | Kollmorgen-Corp-Device |
| 21. | Kollmorgen-Servotronix-Device |
| 22. | Leica-Biosystems-Device |
| 23. | Leica-Microsystems-Device |
| 24. | Cepheid-Device |
| 25. | Draeger-Device |
| 26. | Draeger-Medical-Device |
| 27. | Draeger-Medical-Systems-Device |
| 28. | Fluke-Device |
| 29. | Fluke-Biomedical-Device |
| 30. | General-Electric-Device |
| 31. | GE-Healthcare-Device |
| 32. | Datex-Ohmeda-Device |
| 33. | GE-Medical-System-Device |
| 34. | Imatron-Device |
| 35. | Getinge-Device |
| 36. | Jostra-Device |
| 37. | Getinge-IT-Solutions-Device |
| 38. | Getinge-Sterilization-Device |
| 39. | Honeywell-Device |
| 40. | Honeywell-HomMed-Device |
| 41. | ICU-Medical-Device |
| 42. | Hospira-Device |
| 43. | Physiometrix-Device |
| 44. | Kontron-Device |
| 45. | Kontron-Medical-Device |
| 46. | Maquet-Device |
| 47. | Maquet-Cardiopulmonary-Device |
| 48. | Maquet-CardioVascular-Device |
| 49. | Maquet-Critical-Care-Device |
| 50. | Maquet-Germany-Device |
| 51. | Masimo-Device |
| 52. | Masimo-SET-Pulse-Oximeter |
| 53. | MedAvant-Device |
| 54. | MedAvant-Healthcare-Device |
| 55. | MedAvant-Healthcare-Solutions-Device |
| 56. | Mindray-Device |
| 57. | Mindray-Co-Device |
| 58. | Mindray-DS-USA-Device |
| 59. | Nicolet-Device |
| 60. | Nicolet-Instruments-Device |
| 61. | Nicolet-Neuro-Device |
| 62. | Olympus-Device |
| 63. | Olympus-Image-Systems-Device |
| 64. | Olympus-Soft-Imaging-Device |
| 65. | Omron-Device |
| 66. | Omron-Healthcare-Device |
| 67. | Omron-Tateisi-Device |
| 68. | Panasonic-Device |
| 69. | Panasonic-Healthcare-Device |
| 70. | Philips-Device |
| 71. | Philips-Analytical-X-Ray-Device |
| 72. | Philips-CareServant-Device |
| 73. | Philips-Electronics-Netherlands-Device |
| 74. | Philips-Healthcare-PCCI-Device |
| 75. | Philips-Medical-Systems-Device |
| 76. | Marconi-Medical-Systems-Device |
| 77. | Philips-Medical-Systems-Cardiac-Monitoring-Device |
| 78. | Philips-Oral-Healthcare-Device |
| 79. | Philips-Patient-Monitoring-Device |
| 80. | Philips-SureSigns-Patient-Monitor |
| 81. | Philips-SureSigns-VS3-Patient-Monitor |
| 82. | Philips-SureSigns-VS4-Patient-Monitor |
| 83. | Philips-Personal-Health-Device |
| 84. | Philips-Respironics-Device |
| 85. | Siemens-Device |
| 86. | Acuson-Ultrasound-Device |
| 87. | Siemens-AG-Healthcare-Sector-Device |
| 88. | Siemens-Healthcare-Diagnostics-Device |
| 89. | Siemens-Healthcare-Diagnostics-Manufacturing-Device |
| 90. | SonoSite-Device |
| 91. | Sonosite-MicroMaxx-Ultrasound |
| 92. | St-Jude-Medical-Device |
| 93. | Thoratec-Device |
| 94. | Zimmer-Device |
| 95. | ORTHOsoft-Zimmer-CAS-Device |
| 96. | Zimmer-Elektromedizin-Device |
| 97. | AB-Sciex-Device |
| 98. | ACIST-Medical-Systems-Device |
| 99. | Acteon-Group-Device |
| 100. | ADInstruments-Device |
| 101. | Advance-Sterilization-Products-Device |
| 102. | Advanced-Medical-Information-Device |
| 103. | Advantage-Pharmacy-Device |
| 104. | Aeroscout-Device |
| 105. | Alaris-Inc-Device |
| 106. | Alaris-Medical-Systems-Device |
| 107. | Alcon-Laboratories-Device |
| 108. | Alpinion-Medical-Systems-Device |
| 109. | AmbiCom-Device |
| 110. | American-Telecare-Device |
| 111. | Amgen-USA-Device |
| 112. | Andon-Health-Device |
| 113. | Applied-Biosystems-Device |
| 114. | Applied-Medical-Technologies-Device |
| 115. | ARKRAY-Device |
| 116. | Avizia-Device |
| 117. | Axis-Shield-PoC-Device |
| 118. | B-Braun-Melsungen-Device |
| 119. | Bang-Olufsen-Medicom-Device |
| 120. | Ascensia-Diabetes-Care-Device |
| 121. | Bausch-Lomb-Device |
| 122. | Beacon-Medical-Device |
| 123. | Becton-Dickinson-Device |
| 124. | Bestcare-Cloucal-Device |
| 125. | Bio-logic-Systems-Device |
| 126. | Bio-Rad-Lab-Device |
| 127. | Biodevices-Device |
| 128. | bioMerieux-Italia-Device |
| 129. | Bionet-Device |
| 130. | BIOPAC-Systems-Device |
| 131. | Biosoundlab-Device |
| 132. | Biospace-Device |
| 133. | Biotage-Device |
| 134. | Biotronik-Device |
| 135. | BL-Healthcare-Device |
| 136. | BMT-Medical-Technology-Device |
| 137. | Boston-Scientific-Device |
| 138. | Breathometer-Device |
| 139. | C8-MediSensors-Device |
| 140. | Calypso-Medical-Device |
| 141. | Cambridge-Medical-Robotics-Device |
| 142. | Camtronics-Medical-Systems-Device |
| 143. | CardioMEMS-Device |
| 144. | CardioNet-Device |
| 145. | Cardiopulmonary-Corp-Device |
| 146. | CardioTek-Device |
| 147. | Care-Everywhere-Device |
| 148. | CareCom-Device |
| 149. | CareFusion-Device |
| 150. | CarePredict-Device |
| 151. | Carestream-Health-Device |
| 152. | CareTech-Device |
| 153. | CareView-Communications-Device |
| 154. | Celectronic-eHealth-Device |
| 155. | Centrak-Device |
| 156. | Cerner-Device |
| 157. | CHG-Hospital-Beds-Device |
| 158. | Chile-School-of-Medicine-Device |
| 159. | CIRTEC-Medical-Systems-Device |
| 160. | CliniComp-Device |
| 161. | Cogent-Healthcare-Systems-Device |
| 162. | Colorado-Med-Tech-Device |
| 163. | Compumedics-Device |
| 164. | Conmed-Linvatec-Device |
| 165. | Convergent-Bioscience-Device |
| 166. | Corometrics-Medical-Systems-Device |
| 167. | Criticare-Systems-Device |
| 168. | Cutera-Device |
| 169. | Cytyc-Device |
| 170. | Dainippon-Pharma-Device |
| 171. | DENTSPLY-Gendex-Device |
| 172. | Diatek-Patient-Management-Device |
| 173. | Dictum-Health-Device |
| 174. | Disetronic-Medical-Systems-Device |
| 175. | Dixtal-Biomedica-Device |
| 176. | Dragerwerk-Device |
| 177. | Durr-Dental-Device |
| 178. | Edwards-Lifesciences-Device |
| 179. | Ellex-Medical-Device |
| 180. | Eppendorf-Device |
| 181. | Etymonic-Design-Device |
| 182. | Essilor-Device |
| 183. | Fisher-Paykel-Device |
| 184. | Fresenius-Medical-Care-Device |
| 185. | Fukuda-Denshi-Device |
| 186. | Gem-Med-Device |
| 187. | GN-ReSound-Device |
| 188. | Haag-Streit-Device |
| 189. | Health-Hero-Device |
| 190. | Health-Life-Device |
| 191. | Heart-Force-Medical-Device |
| 192. | HemoCue-Device |
| 193. | Heraeus-Noblelight-Device |
| 194. | Hidea-Solutions-Device |
| 195. | Hill-Rom-Device |
| 196. | Hitachi-Aloka-Medical-Device |
| 197. | Hoana-Medical-Device |
| 198. | Home-Skinovations-Device |
| 199. | HORIBA-Medical-Device |
| 200. | Huntleigh-Healthcare-Device |
| 201. | ICU-Scandinavia-Device |
| 202. | Imricor-Medical-Systems-Device |
| 203. | Indiana-Life-Sciences-Device |
| 204. | InnerSpace-Device |
| 205. | Innomed-Medical-Device |
| 206. | INSIDE-Technology-Device |
| 207. | INTEGRA-Biosciences-Device |
| 208. | Integra-LifeSciences-Device |
| 209. | Integrated-Medical-Systems-Device |
| 210. | Intel-GE-Care-Innovations-Device |
| 211. | Interacoustics-Device |
| 212. | Intuitive-Surgical-Device |
| 213. | Invivo-Device |
| 214. | Ivoclar-Vivadent-Device |
| 215. | Ivy-Biomedical-Device |
| 216. | JASCO-Device |
| 217. | JCT-Healthcare-Device |
| 218. | Johnson-Johnson-Medical-Device |
| 219. | JEOL-Device |
| 220. | Karl-Storz-Imaging-Device |
| 221. | KaVo-Dental-Device |
| 222. | KeyMed-Device |
| 223. | LABiTec-Device |
| 224. | Laerdal-Medical-Device |
| 225. | LI-COR-Biosciences-Device |
| 226. | LifeSync-Device |
| 227. | LRE-Medical-Device |
| 228. | MDS-SCIEX-Device |
| 229. | MEDAV-Device |
| 230. | Mediana-Device |
| 231. | Medicis-Device |
| 232. | Medicore-Device |
| 233. | Medison-X-Ray-Device |
| 234. | Medrad-Device |
| 235. | Medtronic-Diabetes-Device |
| 236. | Mennen-Medical-Device |
| 237. | Micropoint-Biotechnologies-Device |
| 238. | MIR-Medical-International-Research-Device |
| 239. | MOCACARE-Device |
| 240. | Molecular-Devices-Corp-Device |
| 241. | Mortara-Instrument-Device |
| 242. | MX-Imaging-Device |
| 243. | NDS-Surgical-Imaging-Device |
| 244. | Networked-Robotics-Device |
| 245. | Neural-Image-Device |
| 246. | NIDEK-Device |
| 247. | Nihon-Kohden-Device |
| 248. | Nipro-Diagnostics-Device |
| 249. | Nonin-Medical-Device |
| 250. | Novartis-Pharma-Device |
| 251. | Novo-Nordisk-Device |
| 252. | Onyx-Healthcare-Device |
| 253. | Optimedical-Systems-Device |
| 254. | Ortivus-Medical-Device |
| 255. | Oticon-Device |
| 256. | Otsuka-Electronics-Device |
| 257. | Pacific-Biosciences-Device |
| 258. | PaloDEx-Device |
| 259. | Palomar-Medical-Device |
| 260. | Peerbridge-Health-Device |
| 261. | Perkin-Elmer-Device |
| 262. | Pharma-Smart-Device |
| 263. | Phonak-Communications-Device |
| 264. | Physio-Control-Device |
| 265. | Planmeca-Oy-Device |
| 266. | Pointe-Conception-Medical-Device |
| 267. | Power-Medical-Interventions-Device |
| 268. | Midmark-Progeny-Device |
| 269. | Proteus-Digital-Health-Device |
| 270. | Quantum-Medical-Imaging-Device |
| 271. | Radisys-Device |
| 272. | Radiometer-Medical-Device |
| 273. | Rauland-Borg-Device |
| 274. | ResMed-Device |
| 275. | Resurgent-Health-Medical-Device |
| 276. | RF-Surgical-System-Device |
| 277. | Roche-Diagnostics-Device |
| 278. | ScottCare-Device |
| 279. | Secure-Care-Device |
| 280. | SenTec-Device |
| 281. | Senticare-Device |
| 282. | Shenzhen-Homecare-Device |
| 283. | Shenzhen-Lifesense-Medical-Device |
| 284. | Shimadzu-Device |
| 285. | SHL-Telemedicine-Device |
| 286. | Sigma-International-Medical-Device |
| 287. | Sirona-Dental-Systems-Device |
| 288. | Smiths-Medical-Device |
| 289. | Soredex-Device |
| 290. | Spacelabs-Healthcare-Device |
| 291. | Spectrum-Medical-Limited-Device |
| 292. | Sphere-Medical-Device |
| 293. | Starkey-Labs-Device |
| 294. | Stratec-Biomedical-Device |
| 295. | Stryker-Device |
| 296. | Sysmex-Device |
| 297. | Tecan-Systems-Device |
| 298. | Terumo-Device |
| 299. | Thermo-Fisher-Scientific-Device |
| 300. | Tiba-Medical-Device |
| 301. | Tokyo-Boeki-Medisys-Device |
| 302. | Toyo-Medic-Device |
| 303. | tPlus-Medical-Device |
| 304. | Translogic-Device |
| 305. | Trendsetter-Medical-Device |
| 306. | Triton-Electronic-Systems-Device |
| 307. | Tunstall-Healthcare-Device |
| 308. | Valtronic-Device |
| 309. | Varian-Medical-Systems-Device |
| 310. | Versamed-Device |
| 311. | Verto-Medical-Solutions-Device |
| 312. | VIASYS-Healthcare-Device |
| 313. | Vigil-Health-Solutions-Device |
| 314. | VitalCARE-Device |
| 315. | Vivonic-Device |
| 316. | Vocera-Communications-Device |
| 317. | Welch-Allyn-Device |
| 318. | West-Com-Nurse-Call-Device |
| 319. | Widex-Device |
| 320. | WL-Gore-Device |
| 321. | Zoe-Medical-Device |
| 322. | ZOLL-Lifecor-Device |
| 323. | DICOM-Client |
| 324. | DICOM-Server |
| 325. | HL7-Client |
| 326. | HL7-Server |
- Mark as Read
- Mark as New
- Bookmark
- Permalink
- Report Inappropriate Content
A couple questions:
(1) Is there an efficient way to group all medical profiles into a logical profile? I see we can filter using the "Healthcare" description. Is there a way to "Select All" at that point and add them to a logical profile?
(2) Are any of these profiles or updates included in the feed service? When an update to the list comes out, does it hurt anything to manually import it all over again, or will duplicates arise?
Thanks,
Dave
- Mark as Read
- Mark as New
- Bookmark
- Permalink
- Report Inappropriate Content
Unfortunately that is a limitation of current ISE version. There is no option to import logical profiles via file or API today. Therefore, the entries must be added manually today. Recommend have a separate tab/window open to compare list while selecting with CTRL key.
These profiles are not part of Feed update. There is a limit to max profiles (2000) and so these are intended fro user by customers interested in specific list of IoT vertical. There were a couple profiles (parent profiles) at root level which were updated to maintain consistent "scoring" across profiles. Anything flagged as Administrator Created or Modified will not be updated by Feed service, although deleting a profile will cause it to auto-revert to default Feed values if one existed. You can re-import profiles, but realize that any change to profiles--deletion/updates/adds--can impact current classifications and access policy in a production deployment. Treat such imports like patch upgrades to avoid unexpected disruption in service. Also recommend verify all profile updates--including Feed--offline before applying to production deployment.
Find answers to your questions by entering keywords or phrases in the Search bar above. New here? Use these resources to familiarize yourself with the community: